Medication review and deprescribing: from theory to implementation in different care settings
Medication review and deprescribing
The increase in life expectancy and the growing availability of effective drugs for the management of chronic diseases are leading to an increase in the number of patients, suffering of multimorbidity, in polypharmacotherapy (regular intake of at least five different active ingredients by patient). When assessing polypharmacotherapy, it is also important to consider self-medication drugs, food supplements and herbal products. According to the latest report on the use of medicines in Italy, 68.1% of users aged 65 years and over have received prescriptions for at least five different substances and more than one in four (28.6%) have taken at least 10 different active ingredients. One of the main consequences of polypharmacotherapy is the increased risk of drug-drug interactions (DDIs) which, in turn, may increase the risk of adverse drug reactions (ADRs). Therefore, it is essential to implement preventive strategies aimed at minimising potentially inappropriate prescriptions (PIP), simplifying complex therapeutic regimes and replacing with safer and more effective alternatives, when possible, pharmacological treatments whose potential ADRs exceed the expected benefits for the patient. This is the context for medication review and deprescribing interventions, methods oriented towards optimising the therapeutic plan through a multidimensional, systematic and periodic evaluation of current pharmacological treatments. To carry out this assessment, the clinical pharmacologist (pharmacist or doctor) considers, for each drug prescribed, several appropriateness parameters, including the indication for use, dosage, DDI risk, ADR risk, anticholinergic burden and the prescribing cascade. Ultimately, in addition to the prescription of unnecessary drugs, undertreatment should also be identified, taking into account the individual patient’s treatment goals, adherence to therapy, clinical condition, life expectancy and preferences. For all these assessments, it is important to work in synergy with the doctor in charge of the patient and with other health professions that may be supportive, such as the hospital pharmacist, the nurse and the psychologist.
Clinical impact of drug-drug interactions and adverse drug reactions
Drugs that are frequently prescribed inappropriately include proton pump inhibitors, non-steroidal anti-inflammatory drugs, benzodiazepines, vitamin D, antipsychotics and statins. Numerous scientific evidences suggest that the number of drugs taken by the patient is directly proportional to the risk of ADRs (even multiple ones). A recent meta-analysis of observational studies that assessed the prevalence of ADRs in the primary care setting reported an average prevalence ranging from 8.3% to 20.4%. In the context of nursing homes, where most patients are on polypharmacotherapy, the risk of ADRs is even more related to potentially inappropriate prescriptions, the prevalence of which in this population is around 50%. Several studies indicate that about 10% of unscheduled hospitalisations in geriatrics are caused by ADRs and that ADRs may occur during hospitalisation in 25% of patients over 65 years of age. A recent systematic literature review reported that preventable in-hospital ADRs account on average for 37.3% of total ADRs. This proportion increases to over 70% in the elderly population. The costs associated with preventable ADRs occurring in the course of hospitalisation range from a minimum of 2,851.00 euro to a maximum of 9,015.00 euro per patient, with an increase in the duration of hospitalisation, on average, of 8.5 ± 4.2 days. In addition, a great deal of evidence from all care settings shows that reducing the number of PIPs taken by the patient reduces the risk of ADRs and related consequences, such as emergency room admissions, prolonged hospitalisation and increased healthcare costs.
Organisation of a medication review and deprescribing service
In October 2023, the ‘Intersociety document on the implementation of medication review and deprescribing in various care setting’ was published by a working group involving the main Italian scientific societies operating in the fields of pharmacology, geriatrics, internal medicine and general medicine. The publication - 30 pages divided into 8 chapters and 3 infographics - describes the essential elements and operational strategies for the implementation of medication review and deprescribing interventions in different care settings (general medicine, hospital, nursing homes, long-term care and palliative care).
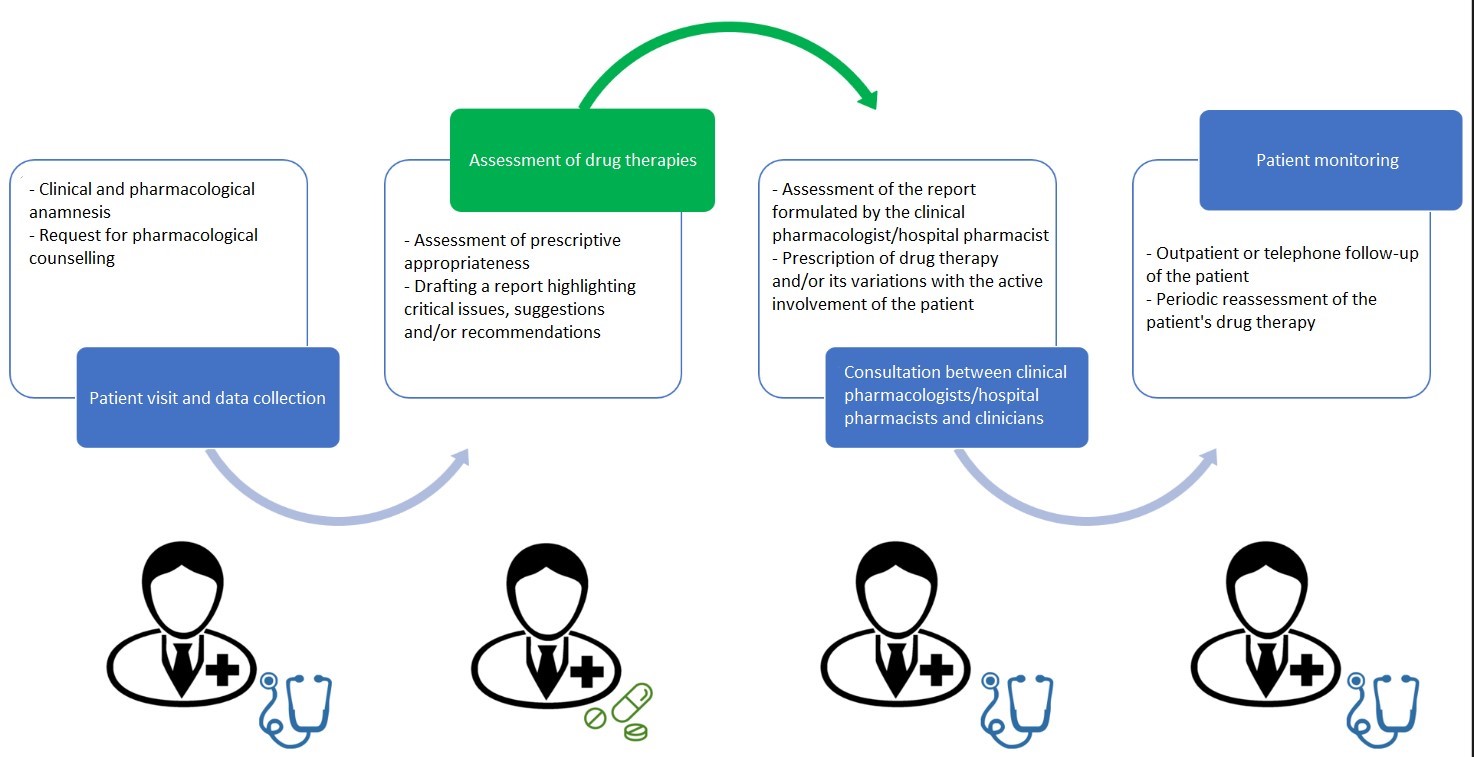
The medication review and deprescribing process has four phases (Figure 1), each with specific objectives and actions:
- Patient examination and data collection: the attending physician takes a thorough clinical and pharmacological history. The clinical anamnesis is based on the assessment of anthropometric parameters, liver and kidney function, comorbidities, laboratory and instrumental tests, fragility indexes and functional assessment scales. The pharmacological history requires a complete list of drugs taken by the patient and evaluation of dosage, frequency, duration, therapeutic adherence, as well as other considerations such as efficacy and adverse reactions.
- Assessment of drug therapies: the clinical pharmacologist assesses the appropriateness of each drug, taking into account the risk of potential DDIs and ADRs (Table 1), the presence of PIPs and potential prescribing cascades. This step also includes the analysis of drugs with a high anticholinergic burden, the use of contraindicated drugs and the evaluation of the benefit-risk ratio.
- Comparison between appropriately trained clinical pharmacologists/hospital pharmacists and prescribing physicians for a shared decision: on the basis of the consultation report, after possible discussion also by telephone, the prescribing physician decides whether or not to make changes to the drug therapy, actively involving the patient or his or her caregiver in the decision-making process. Therapy changes are then documented to ensure continuity of care in the different care settings.
- Monitoring: the last phase consists of monitoring the patient’s adherence to the new therapy and the occurrence of emerging symptoms attributable to changes in therapy, the patient’s self-management of medication and the identification of new ADRs. Depending on the setting, this phase may involve outpatient follow-up or telephone home monitoring, and involves several healthcare professionals to ensure an integrated approach to patient management.
In summary, the medication review and deprescribing process is a systematic, multi-step approach aimed at optimising the use of medication in patients while minimising the risks associated with polypharmacotherapy, especially in vulnerable populations such as the elderly.
Figure 1. Organisation of a medication review and deprescribing service.
The experimental study in AOUI Verona
In the light of current knowledge, it does not appear that any hospital in Italy has implemented a systematic and multidisciplinary procedure similar to the one described, which envisages the active involvement of clinical pharmacologists. The Verona Integrated University Hospital, involving the Operative Units of Clinical Pharmacology, Geriatrics B and Internal Medicine C, will propose to the Ethics Committee a protocol for an experimental investigation aimed at re-evaluating drug therapies in elderly patients admitted to the above-mentioned wards. This study envisages the inclusion of approximately 1,500 patients per year, selected on the basis of the following criteria: age over 65 years, administration of at least five drugs on a chronic basis and life expectancy of at least six months.
Table 1. Classes of drugs and criticalities most frequently requiring re-evaluation in elderly patients, with their indications and main adverse reactions to be monitored.
Pharmacological class | Indication | Adverse reactions / adverse events |
Opioid analgesics | Chronic pain | Drowsiness, nausea, constipation, respiratory depression from improper and/or unattended prescription, dependence |
Antiarrhythmics | Cardiac arrhythmias | Bradycardias, arrhythmias, electrolyte imbalances |
Anticoagulants | Thrombotic or ischaemic-based diseases | Haemorrhages, thrombosis |
Antidepressants | Depression, mood swings | Drowsiness, gastrointestinal disturbances and weight gain |
Antidiabetics | Diabetes | Hypoglycaemia, weight gain/loss, gastrointestinal disorders, peripheral oedemas, urinary tract infections |
Non-steroidal anti-inflammatories | Phlogistic states, pain | Gastrointestinal ulcers, gastrointestinal bleeding and acute renal failure |
Antihypertensives and Diuretics | Arterial hypertension | Electrolyte imbalances, dehydration, acute renal failure, hypotension, dizziness, pre-syncope, syncope |
Antipsychotics | Psychiatric disorders | Weight gain, sedation, memory loss and involuntary movements |
Benzodiazepines | Insomnia, anxiety | Daytime sleepiness, falls, confusion, dependency, paradoxical effect (increased anxiety level, insomnia, irritability, aggravation of seizures for epileptics) |
Bisphosphonates, vitamin D and calcium | Fracture prevention | Gastrointestinal complaints, muscle pain, headaches and atypical fractures |
Proton pump inhibitors | Gastric ulcer, gastroesophageal reflux | Diarrhoea, headaches, increased risk of gastrointestinal infections, kidney failure, bone fractures, vitamin B12 and other nutrient deficiencies, lung infections |
- Italian Medicines Agency (AIFA). National report on medicines use in Italy - Year 2021 of the Italian Medicines Agency, 2021.
- Crisafulli S, Poluzzi E, Lunghi C, et al. Deprescribing as a strategy for improving safety of medicines in older people: clinical and regulatory perspective. Front Drug Saf Regul 2022;2:1011701. DOI:10.3389/fdsfr.2022.1011701.
- Intersociety paper on the implementation of medication review and deprescribing in different care settings. Published October 2023. Academy of Geriatrics (AG), Federation of Associations of Hospital Internist Managers Internal Medicine (FADOI), Italian Society of Palliative Care (SICP), et al. Accessed February 4, 2024. https://sif2022-production.s3.amazonaws.com/uploads/attachment/file_it/2...
- Ant D, Sultana J, Cutroneo PM, et al. The economic burden of preventable adverse drug reactions: a systematic review of observational studies. Expert Opin Drug Saf 2018;17(7):681-695. DOI:10.1080/14740338.2018.1491547.
- Onder G, Vetrano DL, Palmer K, et al. Italian guidelines on management of persons with multimorbidity and polypharmacy. Aging Clin Exp Res 2022;34(5):989-996. DOI:10.1007/s40520-022-02094-z.
Massimo Carollo, Salvatore Crisafulli, Anna Forti, Gianluca Trifirò
Department of Diagnostics and Public Health, University of Verona, Verona