Antihypertensive drugs in pregnancy
The occurrence of hypertension in pregnancy is now more frequent than in the past, due to the increase in maternal age at delivery and related concomitant chronic diseases.1 Hypertension in pregnancy may represent a serious obstetric emergency and is among the leading causes of maternal and neonatal mortality and morbidity.2-3
The recent 2020 Italian Preeclampsia Association (AIPE) guidelines4 classify hypertensive disorders in pregnancy into:
- Hypertension diagnosed before 20 weeks, which includes:
- chronic hypertension
- white coat hypertension
- masked hypertension (detectable only by 24-hour Holter monitoring)
- Hypertension diagnosed after 20 weeks that includes:
- gestational hypertension
- gestational hypertension
- preeclampsia.
In Italy, the frequency of pregnancies complicated by hypertension varies from 5 to 7% with an incidence of preeclampsia of 1-2% of the total number of pregnant women and 20-25% of the total number of hypertensive pregnant women.5-6
Specifically, chronic hypertension affects up to 5% of pregnancies, is more common in the black race, and its prevalence increases with increasing maternal age (0.6%-2% between 18 and 29 years of age and 4.6%-22.6% between 30 and 39 years of age.7 Gestational hypertension complicates approximately 4% of all pregnancies and 15% of twin pregnancies in Western populations.7
The proportion of women with chronic hypertension is of considerable importance because the correct classification of the patient in the preconception phase is one of the best strategies for prevention of maternal-fetal risk.8 Pregnancy planning and proper referral of the patient to a multidisciplinary team allows identification of the etiology of hypertensive disease and recommendation of appropriate laboratory and instrumental tests.
The evaluation of drug therapy is a fundamental step of counseling: the prescription of the most effective and safe antihypertensive therapy in pregnancy allows to improve maternal-fetal outcome and it is therefore important that the clinician knows how to choose the appropriate drug for each patient.9
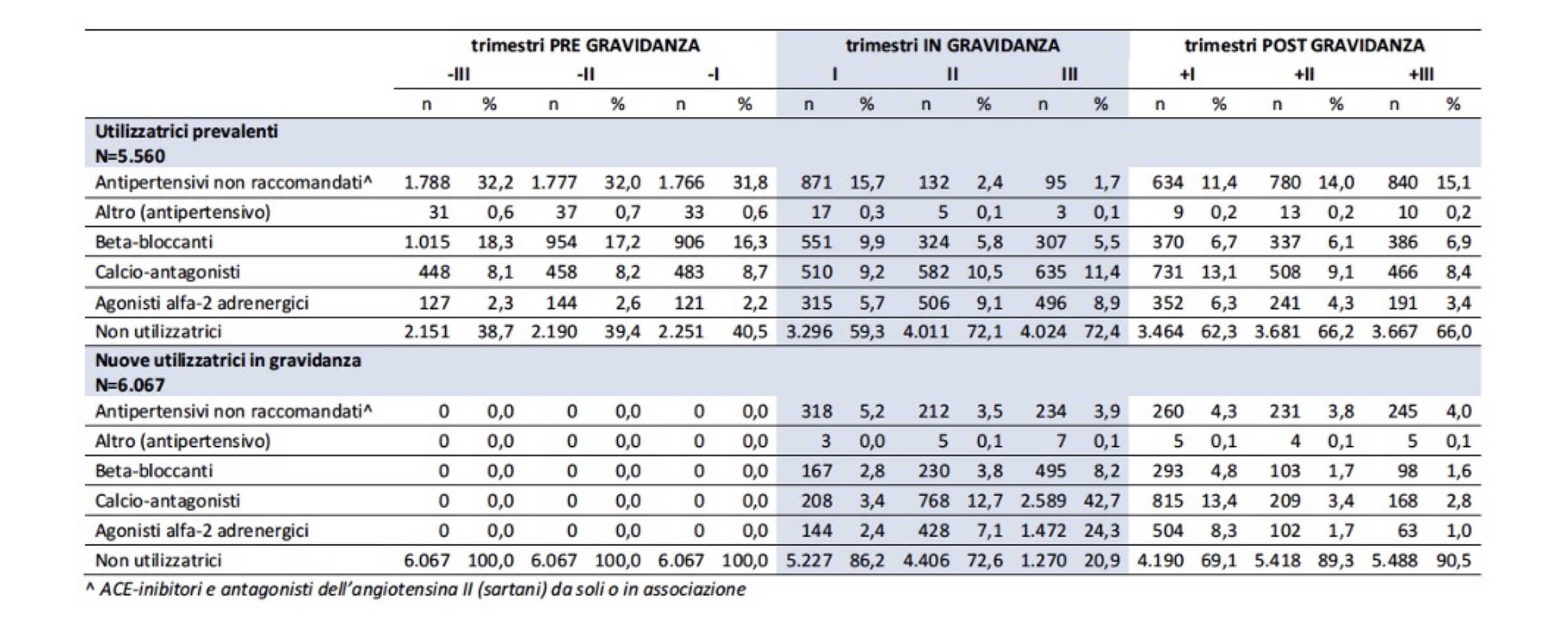
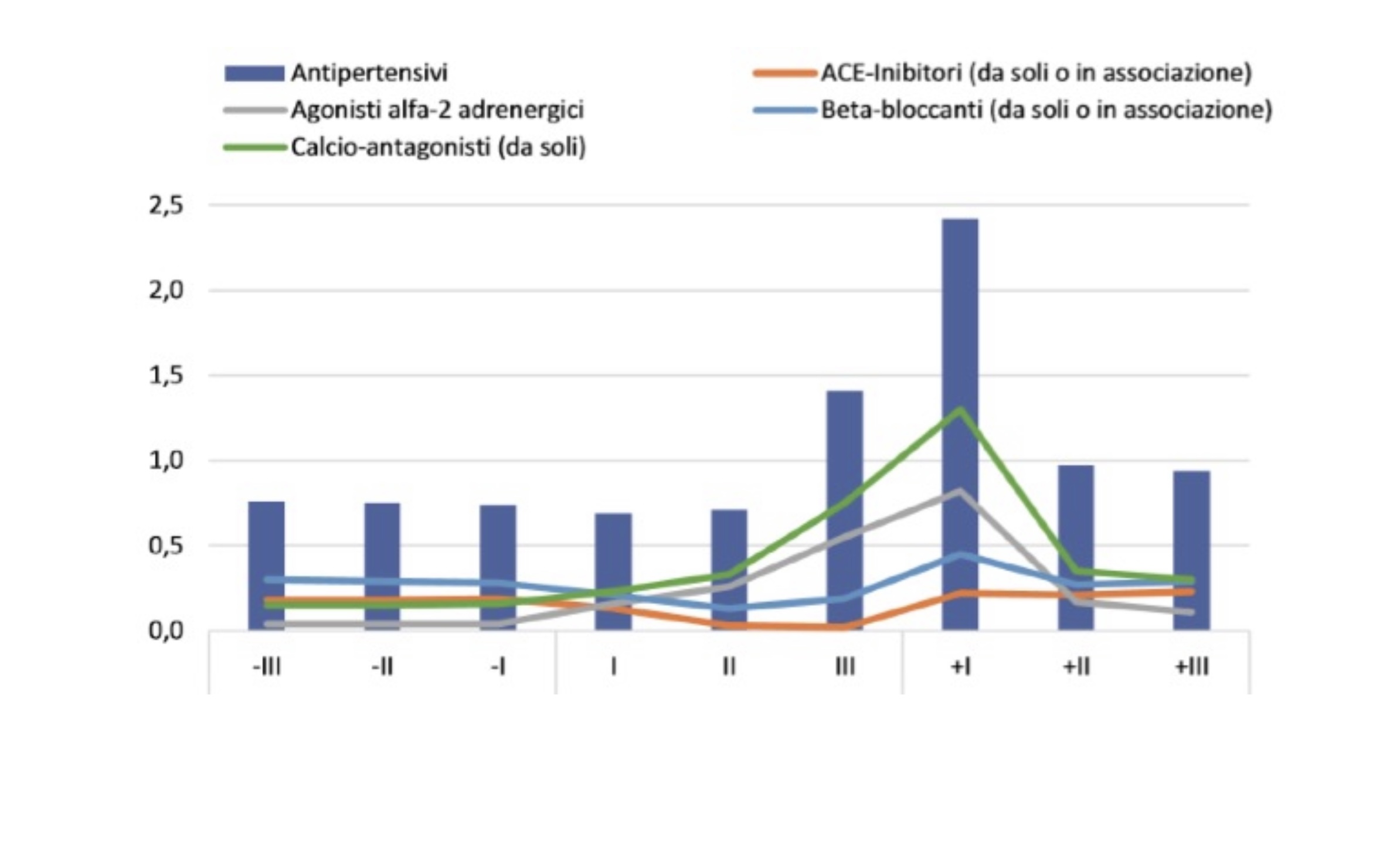
In the “National Report on the Use of Medicines in Italy”, edited by the National Observatory on the Use of Medicines (OsMed),10 the Italian Drug Agency (AIFA) describes the prescribing profile of the various classes of antihypertensives in the trimesters before, during and after pregnancy (Table 1 and Figure 1).
Table 1. Prescribing profile of major antihypertensives in the trimesters before, during, and after pregnancy10
Figure 1. Prescribing prevalence of hypertensive classes before during and after pregnancy10
The prevalence of use of antihypertensives in the preconception period stands at 1.2%, reaching an overall percentage of around 2% during pregnancy, and then rising to 2.9% in the months following delivery (Figure 1). Considering the use by trimesters, the prevalence of use remains almost constant in the pre-pregnancy period, fluctuating between 0.76 and 0.74%, decreases slightly in the first trimester of pregnancy (0.69%) and then begins to increase, going from 0.71% in the second trimester to 1.41% in the third trimester, reaching a peak of 2.4% in the first three months after delivery. Thereafter, the prevalence of use declines sharply, although it does not reach the levels observed in the pre-pregnancy period.10
From the prescribing profiles illustrated in the Report, it can be seen that a proportion of women with drug treated chronic hypertension abandon therapy at a positive pregnancy test, while a portion are exposed to drugs that are not recommended. During pregnancy, the prevalence of prescription decreases slightly from the preconception phase to the first trimester, and then increases progressively in the second and third trimesters. This trend reflects, as indicated by the analysis of the data according to the distinction between prevalent and new drug users, the interruption of therapy by hypertensive women in the first trimester and the increase in use during pregnancy in women in whom a hypertensive disorder is diagnosed.
Interruption of therapy affects a significant proportion of patients; among the prevalent users, probably mostly hypertensive, the proportion of non-users of drugs in the trimesters before pregnancy varies from 38.7 to 40.5%, a proportion that instead stands at 59% in the first trimester (Table 1): hypertensive patients without therapy are exposed to a high risk of hypertensive crisis, related to the non-take of drugs in pregnancy. This figure probably reflects poor pregnancy planning and therefore a loss of the opportunity to carry out preconception counseling, which is particularly attentive to the chronic pathology present and its treatment.
Analyzing the prescriptions of the different classes of antihypertensives in the first trimester, it is possible to observe that the abandonment of therapy by women defined as prevalent users concerns drugs not recommended in pregnancy (Table 1).
According to the “OSMED 2020 National Report on the Use of Drugs in Italy”, the most widely used category of antihypertensives in the Italian hypertensive population is ACE inhibitors,11 drugs not of choice in pregnancy, which expose the unborn child to significant teratogenic and fetotoxic risk.
In Table 1, the share of prevalent women who continue to use these drugs is probably partly attributable to their as yet unknown pregnancy status. The trend in the flow of prescriptions shows that 60% of prescriptions for non-recommended drugs are concentrated in the first trimester, falling to 2% in the following trimesters and rising to 11% after delivery. In new-user women, i.e., patients with a diagnosis of hypertension in pregnancy, the prescription of nonrecommended drugs is between 4 and 5% in both pregnancy and postpartum.
Table 2 shows the teratogenic and/or fetotoxic risk profile of the main therapeutic categories prescribed according to the OSMED report, flanked by their active ingredients. Drugs with a prevalence of use in pregnancy
Table 2. Risk profile of major antihypertensives prescribed in pregnancy
| Therapeutic categories | Active substances | TGA Class. | Reprotox* | Briggs* | Schaeffer* | AIPE drug of choice |
| ACE inhibitors | Benazepril Captopril Enalapril Fosinopril Lisinopril Moexipril Quinapril Ramipril Trandolapril | D | In 3.2% of 2nd and 3rd trimester exposures typical fetopathy (renal damage, oligohydramnios, pulmonary hypoplasia, joint contracture, cranial suture enlargement, vena cava thrombosis). Reported increased risk of cardiac and central nervous system malformations in the first trimester, not confirmed by other studies | Risk of feto-neonatal toxicity from exposures in 2nd and 3rd trimesters: oligohydramnios, fetal growth restriction, persistence of ductus arteriosus, pulmonary hypoplasia, hypocalvaria, joint contracture. Newborn: hypotension and anuria | Contraindicated in pregnancy. Recommended substitution with antihypertensives of choice. For first trimester exposures, detailed fetal ultrasound with possible echocardiogram is recommended. Not indicated invasive procedures or termination of pregnancy. For third trimester exposures, fetal ultrasound monitoring is recommended | |
| Sartans | Candesartan Eprosartan Irbesartan Losartan Olmesartan Telmisartan Valsartan" | D | In 29% of 2nd and 3rd trimester exposures typical fetopathy (renal damage, oligohydramnios, pulmonary hypoplasia, joint contracture, cranial suture enlargement, vena cava thrombosis) | Risk of neonatal fetotoxicity as for ACE inhibitors | ||
| Alpha 2 adrenergic agonists | Alfa-metil dopa | A | Used in the treatment of hypertension in pregnancy | Compatible. Not embryo-fetal risk and neurocognitive abnormalities | Drug of choice, as well as first-line in the treatment of hypertension | Alfa-methyl dopa |
| Clonidine | B3 | Not of choice in pregnancy. Not reported increased risk (limited number of cases) | No increased risk. Few data in first trimester | Possible use as second-line treatment | ||
| Guanfacine | B3 | Not of choice in pregnancy. Not reported increased risk (limited number of cases) | Not increased risk in limited number of cases. Not of choice | Not recommended due to limited number of cases | ||
| Beta-blockers | Acebutolol Atenolol Betaxolol Bisoprolol Carteolol Carvedilol Esmolol Labetalol Levobunolol Metipranolol Metoprolol Nadolol Nebivolol Penbutolol Pindolol Propranolol Sotalol Timolol | C | Not associated with increased risk for first trimester exposures. Infants born to mothers on therapy may manifest bradycardia, hypoglycemia | Some molecules, especially partial agonists may cause growth restriction for 2nd trimester exposures. In III trimester placental weight reduction. Monitor infants for 24-48 hours to assess for beta receptor blockade symptoms. Effective for treatment of maternal hypertension | Labetalol, propranol, metoprolol are among the drugs of choice. Use of other beta blockers is not an indication for termination of pregnancy. Preterm infant: risk of bradycardia, hypoglycemia, and respiratory problems in exposed mothers | Labetalol |
| Calcium antagonists | Amlodipine Diltiazem Felodipine Flunarizine Isradipine Mibefradil Nicardipine Nifedipine Nimodipine Nisoldipine Nitrendipine Verapamil | C | Can be used as antihypertensives and tocolytics. In animals may interfere with embryonic development. No increased risk for use in 2nd and 3rd trimester | No increase in teratogenic risk. Used in 2nd and 3rd trimester for the treatment of hypertension | No evidence of increased risk of malformations for exposure during the first trimester. Nifedipine and verapamil are of choice in pregnancy in the 2nd and 3rd trimesters | Nifedipine |
In line with the previous article “Prescribing drugs in pregnancy in Italy”,12 the risk profile assessment has been reported starting from the Australian classification elaborated by the Therapeutic Goods Administration (TGA),13 from the Reprotox Teratology and Reproductive Toxicology database,14 and from some reference texts such as Brigg 15 and Schaeffer16.
Prescriptive appropriateness has as a prerequisite the precise knowledge of the drugs of choice in pregnancy and the teratogenic and fetotoxic risk profile of each class of antihypertensives. Based on these considerations, it was considered potentially useful to report the active substance of choice for each therapeutic category reported, according to the AIPE 2020 guidelines.4
As mentioned previously among the drugs not recommended in pregnancy are ACE inhibitors and sartans. According to some studies, the assumption in the first trimester seems to be associated with a higher incidence of congenital cardiovascular and central nervous system malformations,17 although according to some authors this risk is related to hypertensive pathology and not to ACE inhibitors18-19. In any case, as a precautionary measure, it is advisable from the preconception phase to replace therapy with ACE inhibitors and sartans with alpha-2 adrenergic agonists, of choice in the first trimester, such as alpha methyl dopa. If pregnancy has not been planned, it is recommended that ACE inhibitor therapy be replaced as soon as possible with drugs of choice in pregnancy.
In women exposed to these drugs in the second and third trimesters, careful fetal clinical-ecographic monitoring is required to assess for the possible occurrence of fetopathy, which is characterized by renal failure, oligohydramnios, joint contracture, pulmonary hypoplasia, and vena cava thrombosis.20-21
In the “National Report on the Use of Medications in Pregnancy”, despite the quotas of inappropriateness, most prescriptions show adherence to the guidelines of the major scientific societies: calcium channel blockers, along with alpha-2 adrenergic agonists, appear to be the most prescribed drugs in all trimesters of pregnancy, as well as after delivery (Figure 1). In hypertension in pregnancy, the drug of first choice is alpha methyl dopa, an effective alpha-2 adrenergic agonist.
If pressure is not adequately controlled by alpha methyl dopa, the use of calcium channel blockers or a beta blocker, preferably labetalol, is recommended.4, 22 The latter, although related to some side effects in the newborn (eg, bradycardia, hypoglycemia, respiratory problems), have a recognized efficacy, so their use is recommended.
The calcium channel blocker is also the drug of first choice in puerperium, as it improves renal perfusion and diuresis23 and does not increase the risk of puerperal depression, like alpha methyl dopa24.
In conclusion, it can be said that in Italy the prescriptive appropriateness regarding hypertension in pregnancy is fair and the drugs of choice in pregnancy are known to most clinicians, who use them correctly. The main area of improvement remains the planning of pregnancy of women of childbearing age with hypertensive disease.
Elena Cesari 1, Filomena Fortinguerra2, Valeria Belleudi3, Roberto Leone4, Renata Bortolus5
1 Vittore Buzzi Children’s Hospital, University of Milan
2 Italian Drug Agency (AIFA), Rome
3 Department of Epidemiology, Lazio Regional Health Service, ASL Rome 1
4 Pharmacology Section, Department of Diagnostics and Public Health, University of Verona
5 Master “Pharmacovigilance and drug regulatory disciplines”, University of Verona
- PLoS One. 2017 Oct 17;12(10):e0186287 CDI
- World Health Organization. Beyond the numbers: reviewing maternal deaths and complications to make pregnancy safer. Geneva, 2004.
- Acta Obstet Gynecol Scand 2018;DOI:10.1111/aogs.13415. CDI
- AIPE. I disordini ipertensivi in gravidanza: classificazione, diagnosi e terapia. Raccomandazioni di buona pratica clinica AIPE (Associazione Italiana Preeclampsia) 2020
- Atti Congresso Regionale AOGOI Lombardia, Lodi 1997;31-38.
- Vergani P. 1° Corso “ Ipertensione e gravidanza ”, Lecco, Marzo 2002.
- Gruppo di Studio SLOG. Raccomandazioni di assistenza ipertensione in gravidanza, 2003
- Br Med Bull 2018;128:75-84. CDI
- Le raccomandazioni per le coppie che vogliono avere un figlio. Progetto “Pensiamoci prima” ICBD, CCM 2011
- L’uso dei farmaci in gravidanza. Rapporto nazionale AIFA 2020
- L’uso dei Farmaci in Italia Rapporto Nazionale Anno 2020 – AIFA 2020
- https://www.farmacovigilanza.eu/content/la-prescrizione-dei-farmaci-grav...
- https://www.tga.gov.au/prescribing-medicines-pregnancy-database
- www.reprotox.org
- Drugs in pregnancy and lactation. 10th ed, 2015.
- Drugs during pregnancy and lactation. 3rd ed, 2015.
- N Eng J Med 2006;354:2443-51. CDI
- Reprod Toxicol 2011;31:540-5.
- BMJ 2011;343:d5931.
- J Hypertens 2020;38:133-41.
- N Engl J Med 2006;354:2498-500.
- Hypertension in pregnancy: diagnosis and management. NICE guideline [NG133] 2019.
- Database of Syst Rev 2018;10:CD002252 CDI
- Pharmacother 2020;127:110196. CDI