The bullous eruption of Vanessa
Vanessa is an 80-year-old woman with type 2 diabetes mellitus, so she takes insulin and linagliptin daily. In addition, her chronic home therapy includes warfarin, acetylsalicylic acid, telmisartan, doxazosin, simvastatin and pantoprazole.
The patient comes to the hospital for the onset of a full-body bullous rash associated with intense itching. After an ineffective approach with local steroid therapy, for the suspicion of an adverse reaction to warfarin, the drug was stopped and substituted with enoxaparin. Due to the persistence of symptoms, Vanessa is admitted to the Emergency Medicine Department, where she begins treatment with intravenous steroids and amoxicillin/clavulanic acid orally, together with the application of steroid cream and fat gauzes.
The patient is then transferred to the subacute care department where, through biopsy of two perilesional samples, a diagnosis of bullous pemphigoid is made, which detects the characteristic IgG deposit at the junction between basal membrane and epidermis. For this reason, the systemic steroid therapy is increased and was associated weekly subcutaneous methotrexate. Within a few days, however, the clinical course is further complicated by recurrent febrile episodes, pleural effusion and oral candidiasis. An antibiotic therapy with piperacillin/tazobactam, topical nystatin and fluconazole is then started.
Agranulocytosis is also found, attributed to methotrexate therapy, which is suspended. Treatment with folin and broad-spectrum antibiotics (vancomycin and meropenem) is performed, then morphine and haloperidol therapy is started subcutaneously and the patient is stabilized.
The explanation and risks
Pemphigoid bullous is a rare frequency autoimmune disease characterized by the development of urticarioid plaques and dense subepithelial vesicles.
The mechanisms leading to the onset of bullous pemphigoid are not yet fully understood, but most likely involve antibody-mediated damage directed to two proteins of the epithelial basal membrane. Numerous evidence suggests that the autoimmune reaction may also be triggered by exposure to several classes of drugs, including DPP-4 inhibitors (or gliptins):1-8 in particular the Summary of Product Characteristics of linagliptin reports bullous pemphigoid as a known, but rare, side effect.
In order to investigate the potential signals related to the appearance of hypoglycemia following exposure to glyptins and to compare their reporting rate with that of other oral hypoglycemic agents, a case-to-case analysis of the adverse reactions reported in the pharmacovigilance database managed by the World Health Organization (VigiBase®) was conducted over a wide time interval (2006-2018).
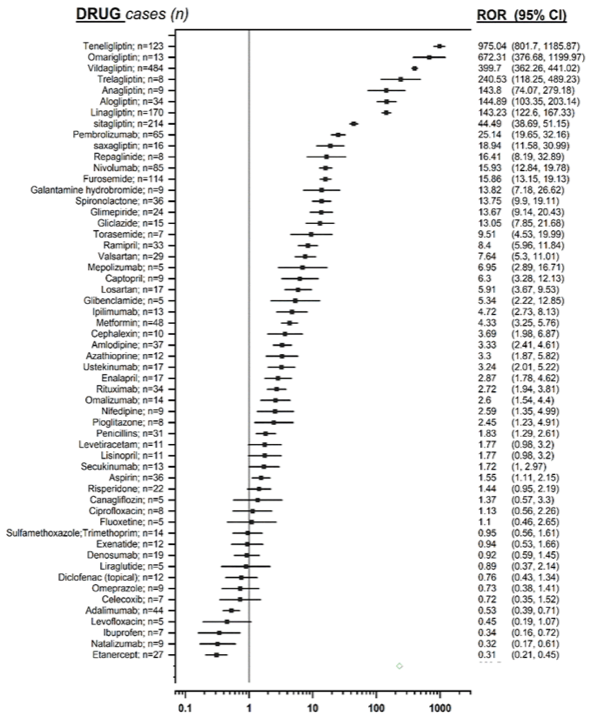
The strength of the association between DPP-4 inhibitors and hypoglycemia was estimated by calculating the Reporting Odds Ratio (ROR) (index of estimated disproportionality, i.e. greater than expected) and its 95% confidence interval (IC95). From this analysis it emerged that glyptins are the drugs associated with the highest frequency of reporting of bullous pemphigoid (ROR: 179.48, 95% confidence limits 166.41-193.58); in particular each single drug belonging to this drug class revealed a significant ROR.
Selecting the cases that report glyptins as the only suspect drugs, the disproportionality values decrease, but maintain the statistical significance.
Finally, a sensitivity analysis was conducted in order to verify the reliability of the previous analysis, comparing the number of cases of glyptin-induced bullous pemphigoid with the number of drug related cases known to be associated to this event. It confirmed a higher signal for glyptins than any other drug (see Figure 1).
In order to explore the pharmacological mechanism underlying the iatrogenic induction of the bullous pemphigoid, the potential role of affinity measures for the biological target that determines the therapeutic effect (DPP-4) and for secondary targets (DPP-2, DPP-8 and DPP-9) was studied; selectivity profiles of each glyptin were also plotted. It was also chosen to study the different distribution volume of the various glyptins, since the postulated hypothesis is that a high-volume distribution glyptin can more effectively access intracellularly located enzymes (DPP-8 and DPP-9).
In order to study whether there was a correlation between the different pharmacological parameter values of the individual glyptins and their estimated ROR measurements, linear regression models were used. From this analysis no significant associations emerged between the different variables studied: the affinity for the enzyme DPP-4 is the one that came closest to statistical significance (p=0.067, R2=0.40),
suggesting that as the affinity for the pharmacological target increases, an increase in the reporting frequency can be expected to some extent.
Although significant, the disproportionality measures identified do not allow to estimate the actual risk, nor to prove any causal association and for this reason future preclinical and clinical studies are necessary to better assess this correlation, including the potential mechanism involved in the etiopathogenesis of this severe disabling disease.
Our analysis results attribute to DPP-4 inhibitors the highest reporting frequency of bullous pemphigoid than any other drug class. It is therefore recommended to carefully monitor all possible symptoms that could be attributable to bullous pemphigoid and, in case of suspected onset, we recommend to stop treatment.
Figure 1. Forest plot showing, on a logarithmic scale, the measures of disproportionality of the drugs analyzed (glyptins are the pharmacological agents associated with the highest ROR values).
To see the graph better click on the image
Giulia Mosini1, Michele Gringeri1, Vera Battini1, Elena Invernizzi1, Olivia Leoni2, Carla Carnovale1, Sonia Radice1
1. Servizio di Farmacovigilanza, U.O. Farmacologia Clinica, Dipartimento di Scienze Biomediche e Cliniche, ASST - Fatebenefratelli-Sacco, Università degli Studi di Milano
2. Centro Regionale di Farmacovigilanza, Regione Lombardia, Milano
- Garcia-Diez I, et al. Bullous pemphigoid induced by dipeptidyl peptidase-4 inhibitors. Eight cases with clinical and immunological characterization. Int J Dermatol 2018;57:810. CDI
- Gaudin O, et al. Gliptin accountability in mucous membrane pemphigoid induction in 24 out of 313 patients. Front Immunol 2018;9:1030. CDI
- Kawaguchi Y, et al. Dipeptidyl peptidase-4 inhibitors-associated bullous pemphigoid: a retrospective study of 168 pemphigoid and 9,304 diabetes mellitus patients. J Diab Investig 2019;10:392. CDI
- Arai M, et al. Bullous pemphigoid and dipeptidyl peptidase-4 inhibitors: a disproportionality analysis based on the Japanese Adverse Drug Event Report Database. Diab Care 2018;41:e130. CDI
- Lee S, et al. Association of dipeptidyl peptidase-4 inhibitor use with risk of bullous pemphigoid in patients with diabetes. JAMA Dermatol 2019;155:172. CDI
- Plaquevent M, et al. Higher frequency of dipeptidyl peptidase-4 inhibitor intake in bullous pemphigoid patients than in the French general population. J Invest Dermatol 2018;139:835-41. CDI
- Garcia M, et al. Dipeptidyl peptidase-IV inhibitors induced bullous pemphigoid: a case report and analysis of cases reported in the European pharmacovigilance database. J Clin Pharm Ther 2016;41:368-70. CDI
- Carnovale C, et al. Bullous pemphigoid induced by dipeptidyl peptidase-4 (DPP-4) inhibitors: a pharmacovigilance-pharmacodynamic/pharmacokinetic assessment through an analysis of the Vigibase®. Expert Opin Drug Saf 2019;18:1099-1108. CDI