Children, drugs and pancreatitis
Paediatric pancreatitis is almost certainly underestimated, and over the last 20 years it has shown an increasing incidence of up to 3-13/100,000 cases per year.1,2 Its classification has been developed by the international INSPPIRE Study Group, which defined the clinical-instrumental diagnostic criteria for acute, recurrent acute and chronic pancreatitis in children.3 While biliary aetiology and alcohol consumption are the main risk determinants in adults, genetic, anatomical, metabolic and toxic factors predominate in pediatrics.4,5 Among the latter, drugs are very important, contributing in more than a quarter of cases and being associated with other risk factors in at least a third of patients.6 This makes it difficult, at times, to define with certainty the pharmacological genesis of pancreatic damage, which, together with the relative rarity of the event and the modest number of available cases, makes it impossible to establish the real incidence of the phenomenon.
Trivedi et al.7 in 2005 and Badalov et al.8 in 2007 proposed diagnostic algorithms to allow the best definition of the cause-effect relationship. The mechanisms of damage can be different: immune-mediated or hypersensitivity, direct drug, inflammatory or mitochondrial toxicity.9 Only three drugs - paracetamol, erythromycin and carbamazepine - have been associated with overdose pancreatitis in children, while most of the reactions appear to be idiosyncratic and therefore unpredictable, non-dose-dependent and with low incidence.
The onset of symptoms is insidious with abdominalgia and non-specific gastrointestinal symptoms (nausea, vomiting, fever, abdominal tension); within a few hours there is an elevation of amylase and lipase of more than three times the normal limit. Once the pharmacological cause is removed, amylases tend to reduce within 48-72 hours while lipases can remain altered for 7-14 days.
The drugs most involved
The drugs most often involved are antiepileptic drugs (first and foremost valproic acid), asparaginase, azathioprine, mesalazine and corticosteroids, but also antibiotics, psychiatric drugs, NSAIDs, antihypertensives, hypoglycemic drugs and antivirals (see Table).4,10
Table. Drugs responsible for pancreatitis according to the classification of Badalov et al.l.8
| Class Ia | Class Ib | Class II | Class III | Class IV |
|---|---|---|---|---|
| α-Methyldopa | Trans-retinoic acid | Chlorothiazide | Alendronate | Ethacrynic acid |
| Valproic acid | Amiodarone | Clozapine | Atorvastatin | Mephenamic acid |
| Azodisalicylate | Azathioprine | Didanosine | Carbamazepine | Ampicillin |
| Bezafibrato | Dexamethasone | Erythromycin | Captopril | Bendroflumethiazide |
| Cannabis | Ifosfamide | Estrogens | Ceftriaxone | Benazepril |
| Carbimazole | Lamivudina | L-asparaginase | Clortalidone | Betametasone |
| Codeina | Losartan | Paracetamol | Cimetidine | Capecitabine |
| Cytosine arabinoside | Linestrenol/methoxyetinylestradiol | Pegaspargasi | Clarithromycin | Cisplatin |
| Dapsone | 6-mercaptopurine | Propofol | Cyclosporine | Colchicine |
| Enalapril | Meglumina | Tamoxifen | Hydrochlorothiazide | Cyclophosphamide |
| Furosemide | Metimazole | Indomethacin | Ciproeptadina | |
| Isoniazide | Nelfinavir | Interferon/ribavirin | Danazol | |
| Mesalazine | Norethindrone/Mestranol | Irbesartan | Diazoxide | |
| Metronidazole | Omeprazole | Isotretinoin | Diclofenac | |
| Pentamidine | Premarin | Ketorolac | Difenoxylate | |
| Pravastatin | Cotrimoxazole | Lisinopril | Doxorubicin | |
| Procainamide | Metolazone | Famciclovir | ||
| Piritonol | Metformin | Finasteride | ||
| Simvastatin | Minocycline | 5-Fluorouracil | ||
| Stibogluconate | Mirtazapine | Fluvastatin | ||
| Sulfamethoxazole | Naproxen | Gemfibrozil | ||
| Sulindac | Paclitaxel | Interleukin-2 | ||
| Tetracycline | Ponatinib | Ketoprofen | ||
| Prednisone | Lovastatin | |||
| Prednisolone | Nitrofurantoin | |||
| Gold salts | Octreotide | |||
| Adrenocorticoid hormones | ||||
| Oxifenbutazone | ||||
| Penicillin |
Often the drug is implicated as concause in the presence of other risk factors, typically genetic (this is the case with thiopurins) or for other underlying diseases (this is the case with Crohn's disease). Out of 271 children with pancreatitis, 55 (25.6%) had a pharmacological cause (10 confirmed, 4 probable and 2 possible).6 20% had epilepsy, 20% leukemia, 11% Crohn's disease and 5% ulcerative colitis. Steroids were the most often involved, followed by valproic acid, mesalazine and cotrimoxazole. In 35% of cases it was a combination of two or more drugs. Compared to other causes of pancreatitis, children with drug-associated pancreatitis had more frequent CT scans, longer hospitalization and greater use of parenteral nutrition. In one study, Cofini et al. reported that 13.5% of pancreatitis cases (7/52) were attributable to drugs, 4 of which were treated with valproic acid.11
Reports of adverse events undergoing valproic acid treatment are numerous in childhood with at least 268 deaths reported from 1977 to 2012.12 58% were severe hepatotoxicity and liver failure, but in 31 cases (11.6%) pancreatitis was present (associated with hepatopathy in only one third of cases). More than half of the children with pancreatitis were in polypharmacy with antiepileptic drugs.
Pancreatitis from valproic acid is not dependent on serum levels of the drug and can occur at any time during therapy with a range of 2 to 84 months of treatment.13,14 The drug should be discontinued and replaced because of the high risk of recurrence, even complicated, of pancreatitis.15 It has been reported that children on monotherapy or antiepileptic polypharmacy (valproic acid, levetiracetam, carbamazepine, oxcarbamazepine, ethosuccimide, lamotrigine, clonazepam) have significantly higher levels of lipase and amylase than controls, even in the absence of clinical signs of pancreatitis. Pancreatic enzyme levels were comparable between those who were in mono and polypharmacy and those who used traditional or second-generation antiepileptic drugs.16 It is therefore likely that several antiepileptic drugs (in addition to valproic acid) have damaging potentials on the pancreas and that the phenomenon is underestimated due to a lack of dosage of serum lipase and amylase in pediatrics.
In patients with chronic inflammatory bowel disease the risk of pancreatitis is 2.1 and 4.3 times for ulcerative colitis and Crohn's disease, respectively. In these patients, the incidence of drug-induced pancreatitis is 15-63% and is responsible for azathioprine, sulfasalazine and 5-ASA with a hypersensitivity reaction dose and non-dependent time. It is usually a mild adverse effect which resolves itself with the discontinuation of treatment. An attempt to reintroduce the suspected drug is possible, provided that the development of serum pancreatic enzymes is closely monitored.17,18
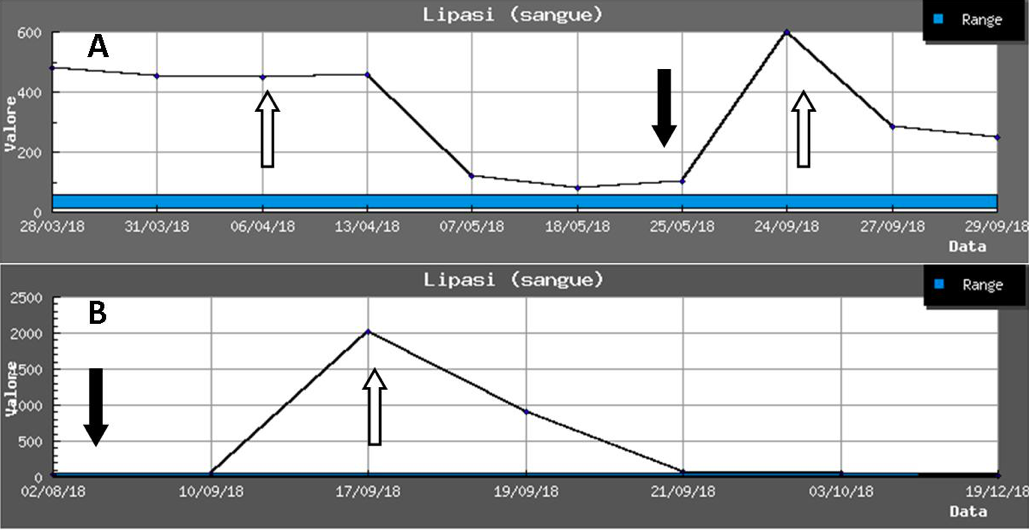
In the field of chronic inflammatory bowel disease, azathioprine is of particular interest, a drug widely used in children and responsible for pancreatitis in 4-7% of patients. Some HLA haplotypes (DQA1*02:01 and DRB1*07:01) appear to carry a significant additional risk factor (odds ratio 2.59) for developing pancreatitis during treatment, with a frequency of 9% in heterozygotes and 17% in homozygotes.19,20 In conclusion, pancreatitis in children is certainly less rare than commonly appreciated and adverse drug reactions represent a proportion of it. A high index of suspicion and a good knowledge of the drugs potentially responsible is necessary to correctly interpret the often non-specific and insidious symptoms. As an example, we report in the Figure the development of lipase in two adolescents with clozapine (A) and azathioprine (B) pancreatitis.
Figure. Modification of lipases in two adolescents in therapy with: A) clozapine, B) azathioprine [black arrows indicate introduction, white arrows indicate drug withdrawal].
Enrico Valletta1, Michele Gangemi2
1 Paediatrics Unit, Hospital G.B. Morgagni - L. Pierantoni, AUSL Romagna, Forlì
2 Paediatrics Unit, Verona
- Gastroenterology 2019;156:1969-78. CDI
- Gastroenterology 2018;155:469-78.e1 CDI
- JPGN 2012;55:261-5 CDI
- JPGN 2016;62:609-17 CDI
- JAMA Pediatr 2016;170:562-9 CDI
- JPGN 2011:53:423-8 CDI
- J Clin Gastroenterol 2005;39:709-16 CDI
- Clin Gastroenterol Hepatol 2007;5:648-61 CDI
- P&T 2013;38:349-51 CDI
- Pancreas 2018;47:1328-36 CDI
- G Chir 2015;36:158-60 CDI
- PloS ONE 2014;9:e108970 CDI
- Pediatrics 2006;118:1660-3 CDI
- J Pediatr Pharmacol Ther 2020;25:256-60 CDI
- Cureus 2015;7:e297 CDI
- Child Neurol Open 2016;3:1-6.
- Pediatr Gastroenterol Hepatol Nutr 2012;15:272-5 CDI
- Pancreas 2019;48:488-95.
- Nat Genet 2014;46:1131-4 CDI