Adverse events from cannabis medical use
Indications for medical use
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main active substances of cannabis sativa and among the pharmacological targets have both cannabinoid receptors (CB1 and CB2), the peptide receptor related to the calcitonin gene, the ligand dependent ion channels of human 5-HT3A receptors and other ion channels and enzymes. The action of THC and CBD on these receptors is responsible for their therapeutic activities, ranging from pain relief to antiemetic, antiepileptic and anti-craving properties.
Italy legally recognized cannabis for medical use in 2006. In 2016 the Italian production of cannabis was authorised and the Florence Military Chemical Pharmaceutical Plant started to grow and treat cannabis in a controlled and standardized environment, according to Good Manufacturing Practice. Therapeutic cannabis can be prescribed as a galenic preparation, as an oil extract, decoction filter and inhalation bags through an authorized device.
In Italy medical cannabis is authorized and reimbursed by the National Health Service for the following medical conditions:
- analgesia in diseases involving spasticity associated with pain (multiple sclerosis, spinal cord injury) resistant to conventional therapies
- analgesia in chronic pain (with particular reference to neuropathic pain) where treatment with nonsteroidal anti-inflammatory drugs or with cortisone or opioids has proved ineffective
- antikinetic and antiemetic effect in nausea and vomiting caused by chemotherapy, radiotherapy, HIV therapies, which cannot be obtained with traditional treatments
- appetite-stimulating effect in cachexia, anorexia, loss of appetite in patients with cancer or AIDS and anorexia nervosa, which cannot be obtained with standard treatments
- ocular hypotensive effect in glaucoma resistant to conventional therapies
- reduction of involuntary movements of the body and face in Gilles de la Tourette syndrome, which cannot be achieved with standard treatments.
Recent evidence shows the positive effects of using medical cannabis both as a first-line treatment and in combination with other therapies in all the clinical conditions mentioned above, identifying cannabis as a viable therapeutic option.
The Pharmacovigilance, Phyto-vigilance and Pharmacoepidemiology research group of the University of Florence, in collaboration with CERFIT (Regional Reference Centre in Phytotherapy) and National Institute of Health, carried out an analysis of the adverse events (AEs) reports for products containing cannabis for medical use, collected in the Italian database of Phyto-vigilance.
The results of the analysis
During the study period (2006-2018) a total of 103 reports of suspected adverse events concerning the use of medical cannabis were collected, of which 61 (59%) were reported by health professionals operating in Tuscany. Eight reports were excluded from the analysis due to the lack of information relevant to the clinical evaluation. The high number of reports from Tuscany, in total 53, can be traced back to the consolidated use of medical cannabis in this region and to the knowledge by physicians and other health care professionals of the procedures for reporting suspected ADR.
In the majority they were women (n=41, 77.3%), with an average age of 62 years. Of all the reports evaluated, 39 (73.6%) were defined as not serious and only in 2 cases the severity of the event was not specified. Most of the adverse events reported (n=45, 84.9%) resulted in a “complete resolution” or “improvement”. Only 2 cases were unresolved at the time of filling in the report form.
Neuropathic and chronic pain were the most reported indication for medical cannabis use. Fibromyalgia and multiple sclerosis were reported in 5 and 2 cases respectively. All patients took oral cannabis at an average dose of 138.5 mg per day, only one patient took cannabis by inhalation. In 38 cases, concomitant therapies were reported, mainly pregabalin, gabapentin and other pain therapies such as paracetamol/codeine or oxycodone/naloxone combinations.
As regards causality assessment, 46 (86.8%) cases were judged “probable” and 5 (9.4%) “possible”. Only one case was defined as certainly associated with the administration of cannabis, while one case was defined as “unclassifiable” due to lack of information.
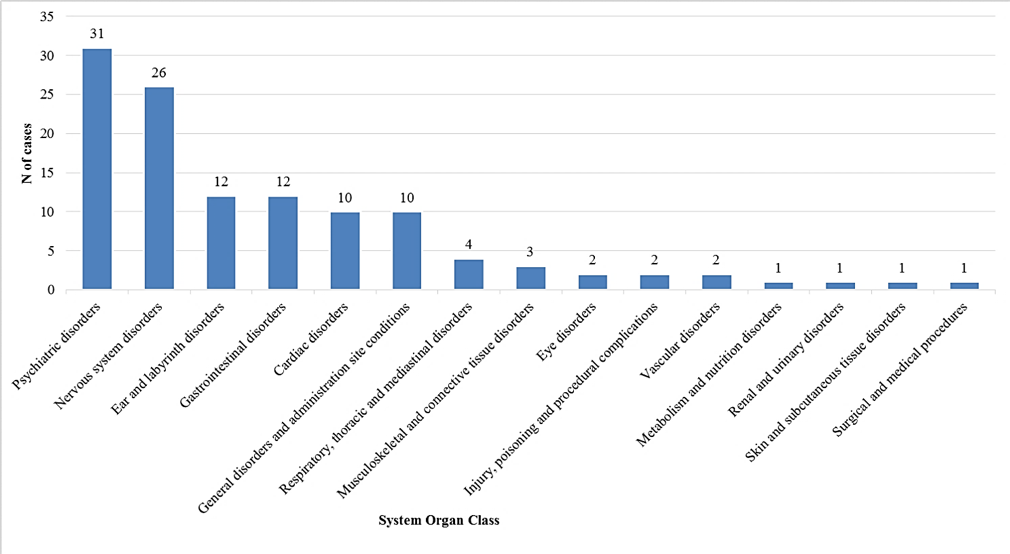
According to the MedDRA classification (Medical Dictionary for Regulatory Activities), the most reported adverse events were: “Psychiatric disorders” (26.3%, n=31 out of 118 adverse events), followed by “Nervous system disorders” (22.0%, 168 n=26), “Ear and labyrinth disorders” (10.2%, n=12), “Gastrointestinal disorders” (10.2%, n=12), “Systemic disorders and conditions related to the place of administration” (8.5%, n=10) and “Cardiac disorders” (8.5%, n=10) (see Figure).
Main adverse events reported according to the MedDRA classification (System Organ Class)1
In practice
This study shows that medical treatment with cannabis is associated with few adverse events, most of which are not serious and that they are completely resolved without any particular consequences for the patient.
As far as our sample is concerned, psychiatric disorders include several adverse events, such as mental confusion, depression and suicidal tendencies, anxiety, acute psychosis, altered body perception and panic attacks. Only two patients reported hallucinations. These psychotic-like transient experiences are described in the literature and, unlike psychotic disorders, are completely resolved in a few hours.
The problems related to labyrinth and ear disorders are due to the presence of CB1 receptors in the vestibular nucleus where, following their activation, the release of glutamate is reduced. This may explain the occurrence of episodes such as loss of balance and labyrinth disorders. In this context, older patients, who are particularly sensitive due to physiological changes, comorbidities, concomitant drug use and cognitive deficits, require special attention.
The most common gastrointestinal disorders were nausea, vomiting and gastritis. They appear to be dose-dependent adverse events mainly related to THC: at low doses, THC is characterized by antiemetic properties, but at high doses it is proemetic.
With regard to adverse cardiovascular events, the following were found in particular: bradyarrhythmia and bradycardia, tachycardia and tachyarrhythmia, supraventricular tachycardia and palpitations, attributable to the presence of CB2 receptors in cardiomyocytes and in the smooth muscles of blood vessels. However, the exact mechanism of the various vascular effects of cannabis has not yet been well characterized.
In most of the cases analysed, patients took at least one other drug in conjunction with cannabis. Adverse events reported in all these cases are common to both cannabis and opioid derivatives, benzodiazepines and antiepileptic agents. Therefore, we believe that these adverse events may be due to a synergistic interaction between cannabis and the most reported concomitant drugs. Moreover, in a clinically complex condition such as the one that often characterizes patients on medical cannabis therapy, considering its excellent analgesic properties, it should be advisable to gradually reduce the administration of concomitant opioids, until it is interrupted when possible.
In our sample we also found 5 cases of “lack of efficacy” (3 patients with chronic neuropathic pain and 2 patients with multiple sclerosis). Since patients with multiple sclerosis were treated with very low doses of cannabis (60 mg per day) for a short time, we assume that their titration phase was still ongoing at the time of reporting. Considering that the duration of medical treatment with cannabis was very heterogeneous among these patients and given the lack of information on individual factors, such as comorbidities, concomitant treatment with other products and genetic factors, it was not possible to assess the causal relationship between the duration of exposure to medical cannabis and the event “lack of efficacy”.
In conclusion prescribers of cannabis should take into account that, especially during the titration phase, treatment may be ineffective and that the onset of adverse psychiatric and gastrointestinal events may increase when cannabis dosages increase too fast and when oral administration is preferred. Particular attention should be paid to the management of elderly patients and those undergoing polypharmacy.
The risks associated with long-term use of medical cannabis are still poorly characterised. Constant medication and phyto-vigilance of adverse events related to cannabis for medical use can improve our knowledge about the profile of cannabis, filling current gaps. In addition, these results could improve current clinical practice and lead to the correct use of cannabis by fully exploiting its therapeutic potential.
Giada Crescioli1, Niccolò Lombardi1, Alessandra Bettiol1, Francesca Menniti-Ippolito2, Roberto Da Cas2, Maria Parrilli3, Martina Del Lungo1,3, Eugenia Gallo4,5, Alessandro Mugelli1, Valentina Maggini4,5, Fabio Firenzuoli5, Alfredo Vannacci1
1 Department of Neuroscience, Psychology, Medicine and Child Health Area - NEUROFARBA, Section of Pharmacology and Toxicology, University of Florence, Florence, Italy
2 National Centre for Research and Preclinical and Clinical Evaluation of Medicines, National Institute of Health, Rome
3 Regional Centre for Pharmacovigilance, USL Toscana Centro, Florence
4 Department of Experimental and Clinical Medicine, University of Florence, Florence
5 Regional Reference Centre in Phytotherapy - CERFIT, Careggi University Hospital (AOUC), Florence
Crescioli G, Lombardi N, Bettiol A, Menniti-Ippolito F, Da Cas R, Gallo E, Mugelli A, Maggini V, Firenzuoli F, Parrilli M, Del Lungo M, Vannacci A. Adverse events following Cannabis for medical use in Tuscany: an analysis of the Italian Phytovigilance database. Br J Clin Pharmacol.2019. CDI